Microbial contamination testing in biopharmaceutical manufacturing
$10 Billion Market Opportunity1
The biopharmaceutical industry is working towards automating the entire manufacturing workflow (Pharma 4.0), where real-time data and artificial intelligence will maximize manufacturing efficiency.
To achieve Pharma 4.0, it is critical to have automated in-process microbial testing, yet no technology is currently meeting the industry’s needs.
This provides an exceptional opportunity for LexaGene Life Sciences and the MiQLab® System.
The biopharmaceutical manufacturing process has significant risks of microbial contamination. The inability to identify a contamination early in the process can result in:
- Loss of product
- Loss of manufacturing time
- Extensive cleaning
- Lost revenue
Larger Bioreactors = Increased Contamination Incident Risk and the Potential Cost of an Incident
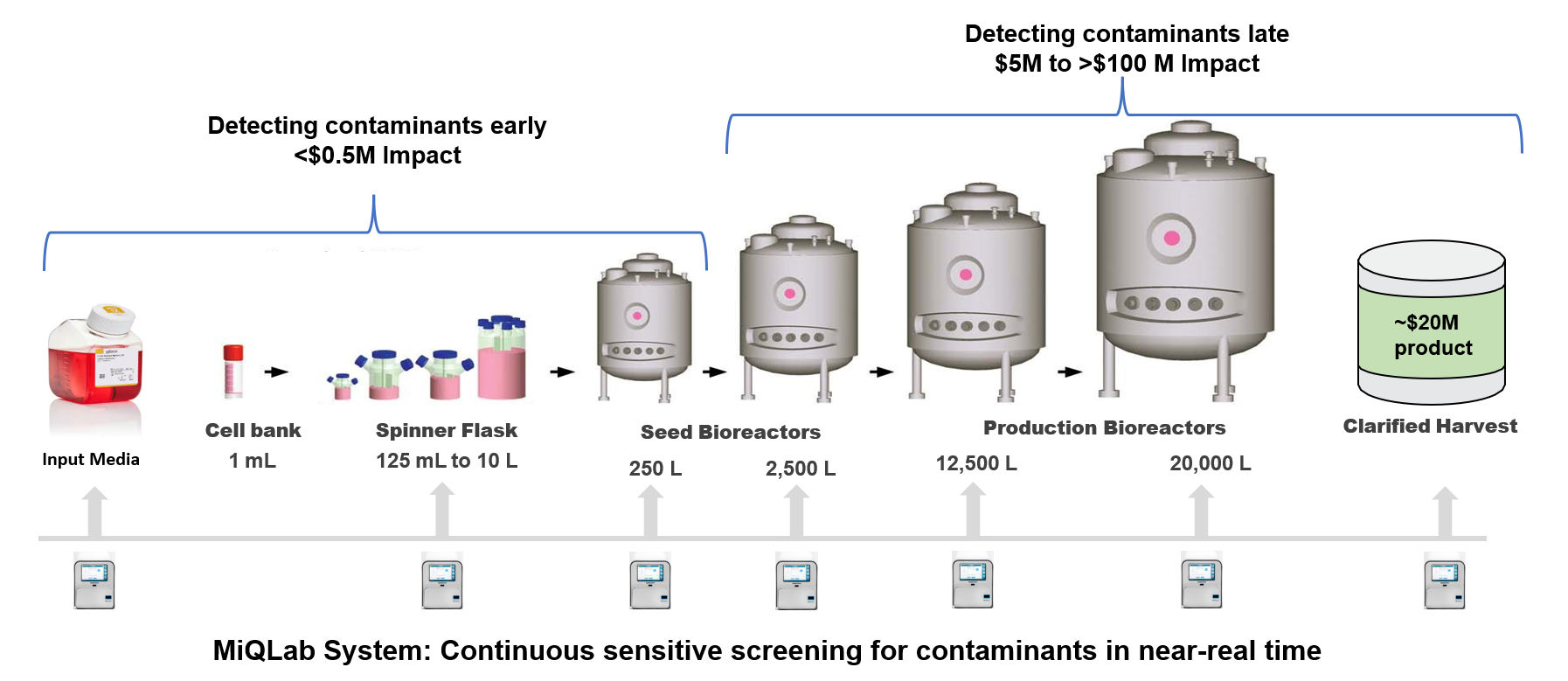
For example, a leading global biopharmaceutical company was fined $175M by the FDA for releasing contaminated product and the impact to the company was likely $100’s of millions more.3
Every major manufacturer has to deal with contamination incidences, but since most are caught before product is released – notification of these incidences never make it into the public domain. Nonetheless, contaminations remain a significant and costly problem for the industry.
Because of their fear of discovering contaminants late in the manufacturing process, two leading biopharmaceutical manufacturers purchased LexaGene’s MiQLab System and expressed great excitement over the potential for the technology to massively disrupt their industry.
LexaGene completed feasibility studies for these customers, which were viewed as very successful. Following these studies, they expressed interest in a revised revision of the technology to better meet the industry’s regulatory requirements.
Fortunately, many of the advantages of the 1st generation MiQLab System can be leveraged in the design of the 2ndgeneration system. This minimizes risk, will speed development work, and will shorten the time to product launch.
Rapid Screening
Our MiQLab System is an automated, PCR system that allows users to test for numerous contaminants onsite, returning results in ~2 hours.
Next Generation Biomanufacturing
Shifting towards the next era of biomanufacturing stands as a top priority for the industry, and in-process microbial testing will play a pivotal role.
Biopharma companies are steadily moving toward intensified, continuous, and automated production of biologics, often referred to as “biopharma 4.0”.
The 4.0 transformation relies on advanced instrumentation, including process analytical technology, or PAT, to achieve this objective.
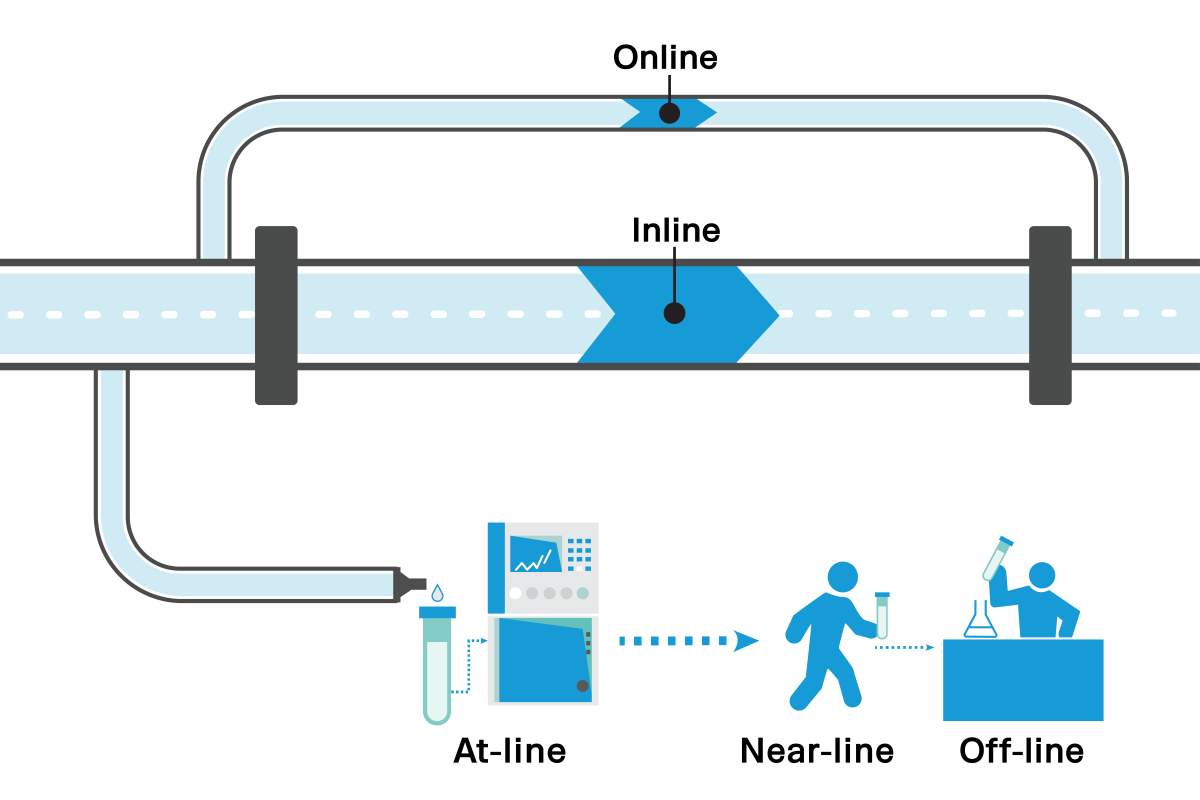
4.0 PAT must enable testing at-line (requires sampling but enables faster turnaround time), on-line (connected to the process line), or in-line (fully integrated into the line). LexaGene’s 2nd generation MiQLab™ System is intended to first be used in near-line settings. As the technology matures, the goal is to move the technology to at-line environments.
A full industry shift will take a number of years, but forward-looking companies have a persistent interest in adopting advanced technology to enable this transformation.
While 4.0-capable analytical techniques are already being implemented to evaluate the properties of a drug itself, 4.0-compatible tools for microbial testing remain a bottleneck and present a compelling opportunity for LexaGene Life Sciences.
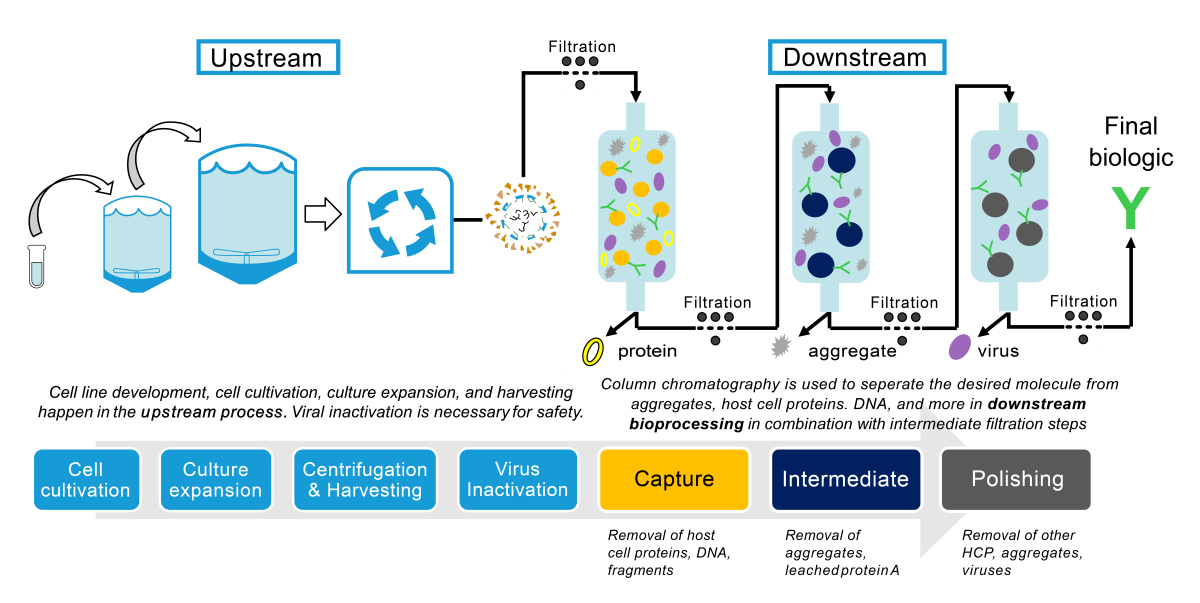
During biopharmaceutical manufacturing, routine microbial contamination screening is essential to ensure a safe product and to maintain manufacturing efficiency.
Within any large biopharmaceutical company, there are multiple departments beyond manufacturing that can benefit from LexaGene’s technology, including: R&D, Process Development, Analytical Development, Process Analytical Technologies, & QA/QC
Learn more about MiQLab